What does a Regulatory Affairs Director do?
The Regulatory Affairs Director oversees all regulatory activities for a company. This position involves working with government agencies to ensure the company complies with all laws and regulations. The director reviews and interprets regulatory requirements. They then develop policies and procedures to meet these requirements. This role requires a deep understanding of industry standards and regulatory changes.
Responsibilities of the Regulatory Affairs Director include leading a team of regulatory professionals. They coordinate with different departments to ensure compliance. The director also represents the company in meetings with regulatory bodies. This position involves preparing reports and documentation. It also requires staying updated on regulatory trends and changes. The Regulatory Affairs Director plays a crucial role in maintaining the company’s reputation and legal standing.
How to become a Regulatory Affairs Director?
A Regulatory Affairs Director plays a key role in ensuring that companies comply with health and safety laws. This role is crucial in industries like pharmaceuticals, medical devices, and food. If you want to become a Regulatory Affairs Director, here is a clear path to follow.
Follow these steps to start your journey:
- Get a relevant degree. Start with a bachelor’s degree in a field like biology, chemistry, or business. This provides the basic knowledge needed for the job.
- Gain experience. Work in roles related to regulatory affairs. This helps build the practical skills needed.
- Pursue a master’s degree. Consider a master’s in regulatory affairs, public policy, or a related field. This can make you more competitive.
- Obtain certifications. Look for certifications in regulatory affairs. These credentials can boost your resume.
- Apply for the right job. Look for openings in companies where you can use your skills. Tailor your resume and cover letter to match the job description.
How long does it take to become a Regulatory Affairs Director?
A Regulatory Affairs Director plays a key role in the pharmaceutical and medical device industries. This role involves ensuring products meet all necessary regulations and standards. Many professionals find this job both challenging and rewarding. The time it takes to reach this position can vary widely.
Most Regulatory Affairs Directors hold a bachelor's degree in a science or health-related field. This often takes four years to complete. Some may pursue a master’s degree, which adds another two years. Gaining experience in regulatory affairs is crucial. This can take three to five years. Experience might come from roles such as a Regulatory Affairs Specialist or Manager. Many directors have at least seven years of relevant experience. Each step builds the necessary skills and knowledge for the job.
Gaining the right experience can lead to promotions and higher responsibilities. Building a strong network in the industry also helps. This combination of education and experience can set someone on the path to becoming a Regulatory Affairs Director. With dedication and hard work, this goal is within reach.
Regulatory Affairs Director Job Description Sample
The Regulatory Affairs Director will be responsible for overseeing the regulatory affairs function of the organization, ensuring compliance with all relevant regulatory requirements and guiding the company through complex regulatory landscapes.
Responsibilities:
- Develop and implement regulatory strategies for new and existing products.
- Ensure compliance with all local, regional, and global regulatory requirements.
- Lead regulatory submissions and interactions with regulatory agencies.
- Collaborate with cross-functional teams including R&D, Quality Assurance, and Marketing.
- Provide regulatory guidance and training to internal stakeholders.
Qualifications
- Advanced degree (PhD, PharmD, JD) in a relevant scientific or legal field.
- Minimum of 10 years of experience in regulatory affairs, with a strong background in the specific industry.
- Proven track record in managing regulatory submissions and interactions with regulatory agencies.
- Strong understanding of regulatory requirements and industry standards.
- Excellent communication and leadership skills.
Is becoming a Regulatory Affairs Director a good career path?
The career path of a Regulatory Affairs Director involves overseeing compliance with laws and regulations. This role requires strong communication and analytical skills. Directors work with government agencies to ensure their company’s products meet all necessary standards. This position offers a mix of office work and field visits.
A Regulatory Affairs Director enjoys many benefits. They have the opportunity to make a significant impact on public health and safety. This role can also lead to good pay and job security. However, it can be demanding. The job often involves strict deadlines and high-pressure situations. Directors must stay updated on changing regulations, which can be challenging. Below is a list of pros and cons to consider for this career path.
Pros:
- Opportunity to impact public health
- Good salary and job stability
- Variety of tasks and challenges
Cons:
- High-stress deadlines
- Constant need to learn new regulations
- Long hours and fieldwork
What is the job outlook for a Regulatory Affairs Director?
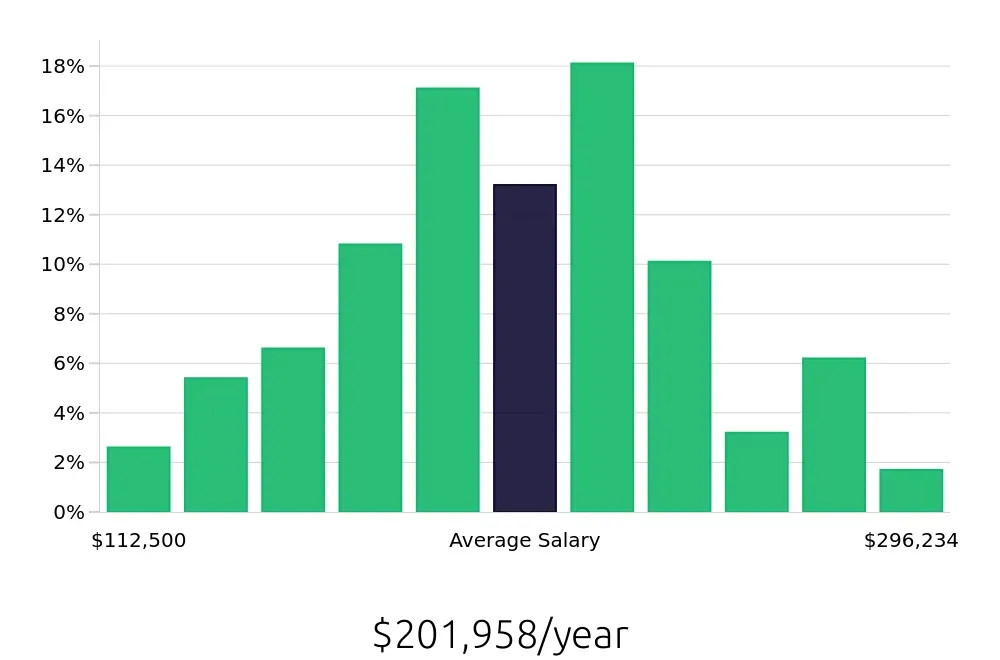
A career as a Regulatory Affairs Director offers promising opportunities for job seekers in the healthcare and pharmaceutical industries. With an average of 94,400 positions available each year, this role is in steady demand. The job outlook is positive, with a projected 3.3% increase in openings from 2022 to 2032, according to the Bureau of Labor Statistics (BLS). This growth highlights the growing importance of regulatory compliance and the need for skilled professionals who can navigate complex regulatory environments.
Professionals in this role can expect a competitive salary, with an average national annual compensation of $146,320, as reported by the BLS. This attractive pay reflects the critical nature of the work, which involves ensuring that companies adhere to government regulations. Hourly compensation averages around $70.35, making this a lucrative career choice. The ability to influence public policy and corporate strategy further adds to the appeal of this role.
For those interested in a dynamic and impactful career, becoming a Regulatory Affairs Director is a smart move. The combination of steady job growth, strong compensation, and the chance to make significant contributions to public health and safety make this an excellent path for ambitious professionals. Aspiring directors should focus on building expertise in regulatory processes and gaining experience in the relevant industry sectors.
Currently 75 Regulatory Affairs Director job openings, nationwide.
Continue to Salaries for Regulatory Affairs Director