What does a Regulatory Affairs Manager do?
The Regulatory Affairs Manager plays a key role in ensuring that a company complies with laws and regulations in the industry it operates. This professional works closely with government agencies to understand new laws and how they affect the company. They prepare and submit necessary documents to get approvals for products or services. This person also communicates with internal teams to ensure all aspects of the business follow legal standards.
This role requires a deep understanding of regulatory environments and an ability to translate complex legal language into actionable steps. The Regulatory Affairs Manager must stay up to date with changes in laws and regulations. They need strong communication skills to liaise between the company and regulatory bodies. This job involves meticulous attention to detail and the ability to manage multiple tasks at once. They often work under pressure to meet strict deadlines. The Regulatory Affairs Manager helps protect the company from legal issues and supports its growth.
How to become a Regulatory Affairs Manager?
Becoming a Regulatory Affairs Manager involves a structured process that combines education, experience, and the right certifications. This role is vital in industries such as pharmaceuticals, biotechnology, and medical devices. A Regulatory Affairs Manager ensures that products meet all regulatory requirements and navigates the complex landscape of regulations.
Here are five essential steps to guide someone on this career path:
- Earn a Relevant Degree: Start with a bachelor’s degree in a related field. Common degrees include biology, chemistry, pharmacy, or business.
- Gain Experience: Work in roles that offer exposure to regulatory processes. Positions in quality assurance, product development, or clinical research are beneficial.
- Pursue Certifications: Consider certifications like the Certified Regulatory Affairs Professional (C-RAP) to enhance credibility and knowledge.
- Develop Key Skills: Focus on building skills such as attention to detail, strong communication, and a deep understanding of regulatory frameworks.
- Network and Apply: Join professional organizations and attend industry events. Apply for positions that match your skills and experience.
How long does it take to become a Regulatory Affairs Manager?
Interested in a career in Regulatory Affairs? Knowing how long it takes can guide your planning. Generally, it takes about four to six years to become a Regulatory Affairs Manager. This timeline often includes getting a bachelor's degree and gaining relevant work experience.
First, a bachelor's degree is essential. Most employers look for degrees in science, health, or business fields. Courses cover topics like biology, chemistry, and law. Internships during studies provide valuable experience. After graduation, new professionals often start in entry-level positions. They may work as Regulatory Affairs Specialists. Gaining experience in this role helps build knowledge and skills needed for a manager position. With about two to four years of experience, professionals can aim for a Regulatory Affairs Manager role. This path leads to a rewarding career in a critical industry.
Regulatory Affairs Manager Job Description Sample
The Regulatory Affairs Manager is responsible for ensuring compliance with all relevant regulations and guidelines, managing interactions with regulatory authorities, and overseeing the regulatory aspects of product development and market authorization. This role requires a deep understanding of regulatory requirements, strategic planning, and excellent communication skills.
Responsibilities:
- Develop and implement regulatory strategies to ensure compliance with local, national, and international regulations.
- Manage interactions with regulatory authorities, including submissions, audits, and inspections.
- Oversee the preparation and submission of regulatory documentation and applications.
- Collaborate with cross-functional teams to ensure regulatory requirements are integrated into product development and lifecycle management.
- Stay updated on changes in regulations and industry trends, and provide recommendations for strategic adjustments.
Qualifications
- Bachelor's degree in life sciences, pharmacy, business administration, or a related field; advanced degree preferred.
- Proven experience in regulatory affairs, preferably within the pharmaceutical, biotechnology, or medical device industry.
- In-depth knowledge of regulatory requirements and guidelines relevant to the industry.
- Strong understanding of regulatory processes and the ability to interpret and apply regulations to business operations.
- Excellent communication, interpersonal, and negotiation skills.
Is becoming a Regulatory Affairs Manager a good career path?
Regulatory Affairs Managers ensure that companies follow the laws and regulations that govern their industry. These professionals work in various sectors, including pharmaceuticals, medical devices, and food. Their main task is to understand and interpret complex regulations. They also make sure that their company complies with these rules. A Regulatory Affairs Manager might prepare documents for regulatory submissions. They might also interact with regulatory bodies to address any concerns.
This career offers several advantages. Regulatory Affairs Managers often enjoy job stability because regulations do not change frequently. They also play a crucial role in ensuring public safety. These managers work with a diverse set of people, from scientists to lawyers. However, this role has its challenges. Regulatory Affairs Managers must keep up with ever-changing laws. They often deal with high-pressure situations. The job can be very detail-oriented, requiring careful attention to every document and process.
Here are some pros and cons to consider:
- Pros:
- Job stability due to consistent regulatory requirements.
- Opportunity to make a difference by ensuring public safety.
- Diverse workplace interactions with various professionals.
- Cons:
- Need to stay updated on frequent regulatory changes.
- High-pressure situations when dealing with regulatory submissions.
- Detail-oriented work that requires careful attention.
What is the job outlook for a Regulatory Affairs Manager?
Regulatory Affairs Managers play a crucial role in ensuring businesses comply with industry regulations. The job outlook for this role is promising, with an average of 94,400 positions available each year according to the Bureau of Labor Statistics (BLS). This role is essential for companies operating in regulated industries, such as pharmaceuticals and medical devices.
The BLS projects a 3.3% growth in job openings for Regulatory Affairs Managers from 2022 to 2032. This steady increase highlights the growing need for professionals who can navigate complex regulatory environments. With advancements in technology and more stringent regulations, companies will continue to require skilled Regulatory Affairs Managers.
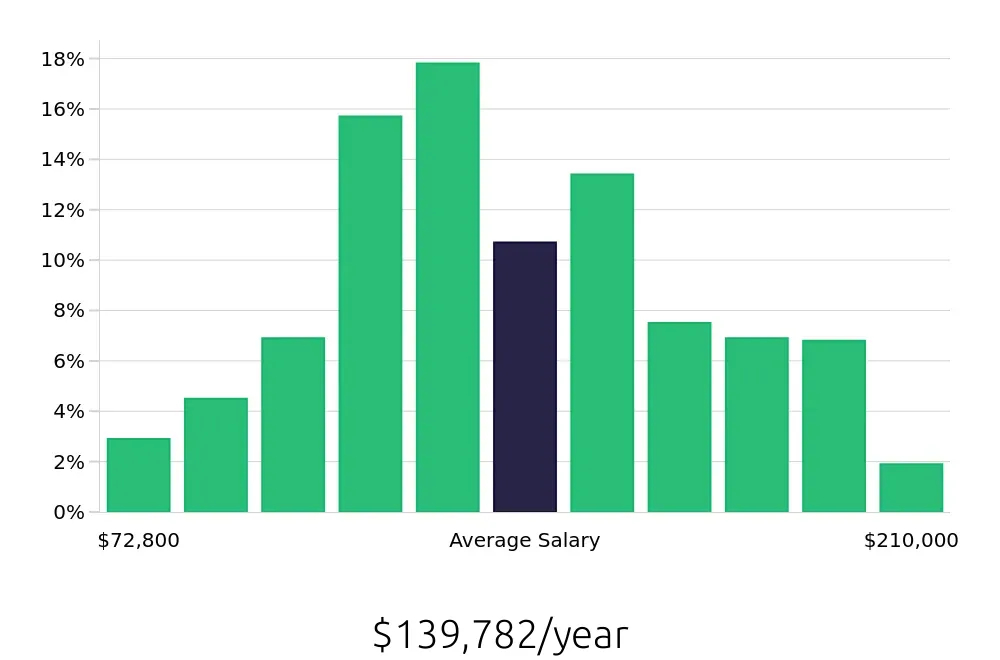
A Regulatory Affairs Manager earns a competitive salary. The average national annual compensation stands at $146,320, with an hourly rate of $70.35, as reported by the BLS. This compensation reflects the specialized skills and knowledge required to excel in this role. Job seekers looking for a stable and well-paying career will find this field attractive.
Currently 95 Regulatory Affairs Manager job openings, nationwide.
Continue to Salaries for Regulatory Affairs Manager