What does a Clinical Research Manager do?
A Clinical Research Manager oversees clinical trials and research projects. This role requires a strong understanding of medical research and clinical trial processes. The manager coordinates with various teams to ensure smooth operations. They manage study timelines, budgets, and resources. This position demands excellent communication and leadership skills.
The Clinical Research Manager also works closely with regulatory bodies. They ensure all research activities comply with legal and ethical standards. This role involves training and mentoring research staff. They help improve the team's skills and knowledge. The manager analyzes data and reports findings to stakeholders. This role is vital for the success of clinical research projects.
Responsibilities include:
- Coordinating with researchers and healthcare professionals
- Managing project timelines and budgets
- Ensuring compliance with regulations
- Training and mentoring research staff
- Analyzing data and reporting results
How to become a Clinical Research Manager?
Becoming a Clinical Research Manager involves a blend of education, experience, and the right skills. This role is crucial in the healthcare industry, overseeing clinical trials and research projects. The path to this position can be clear and rewarding with the right steps.
Firstly, obtaining a solid educational foundation is essential. A bachelor’s degree in a related field such as biology, nursing, or health sciences often serves as a strong starting point. This base provides the necessary knowledge of medical practices and research methodologies. Secondly, gaining practical experience is vital. Working as a Clinical Research Coordinator or in a similar role allows for the application of theoretical knowledge in real-world settings. This experience helps in understanding the day-to-day operations of clinical research.
To advance, one can pursue a master’s degree in clinical research or a related field. This higher education enhances credibility and opens up more advanced roles. Building a professional network within the industry is also key. Attending conferences and joining professional organizations can lead to new opportunities and insights. Lastly, obtaining certifications such as the Certified Clinical Research Professional (CCRP) can further boost a candidate’s resume. This certification demonstrates a commitment to the profession and expertise in clinical research.
The following steps outline a clear path to becoming a Clinical Research Manager:
- Earn a bachelor’s degree in a related field.
- Gain experience by working in clinical research roles.
- Pursue a master’s degree in clinical research or a related area.
- Build a professional network through industry events and organizations.
- Obtain certifications to enhance qualifications.
How long does it take to become a Clinical Research Manager?
Pursuing a role as a Clinical Research Manager requires dedication and a structured educational path. This journey begins with a bachelor's degree in a related field such as biology, chemistry, or health sciences. Most programs take about four years to complete. During this time, students gain essential knowledge about clinical studies and research methodologies.
After earning a bachelor's degree, gaining experience in clinical research is crucial. Many professionals enter the field with roles like clinical research assistants or coordinators. These positions often require 1-2 years of experience. Individuals may also choose to pursue a master's degree in clinical research or a related field. This advanced degree can take an additional 1-2 years. Some employers prefer or require a master's degree for managerial roles. In total, it usually takes about 6-8 years to become a Clinical Research Manager. This includes time spent in education and gaining practical experience. A combination of formal education and hands-on work leads to successful management in clinical research.
Clinical Research Manager Job Description Sample
A Clinical Research Manager is responsible for overseeing and coordinating clinical research projects to ensure they are conducted in compliance with regulatory requirements, ethical standards, and within budget and timelines.
Responsibilities:
- Develop and implement clinical research protocols and study plans in collaboration with cross-functional teams.
- Manage and supervise clinical research staff, including assigning tasks, conducting performance evaluations, and providing training and development opportunities.
- Coordinate with clinical sites, vendors, and other stakeholders to ensure the successful execution of clinical trials.
- Ensure data integrity and compliance with Good Clinical Practice (GCP) guidelines, regulatory requirements, and internal policies.
- Prepare and present study reports, updates, and findings to stakeholders, including senior management and regulatory agencies.
Qualifications
- Bachelor’s degree in a scientific discipline (e.g., Life Sciences, Pharmacy, Nursing, or equivalent); advanced degree preferred.
- Minimum of 5-7 years of experience in clinical research, with at least 2-3 years in a managerial or supervisory role.
- In-depth knowledge of clinical trial methodology, regulatory requirements, and Good Clinical Practice (GCP) guidelines.
- Experience in managing multi-site clinical trials and cross-functional teams.
- Strong leadership, organizational, and project management skills.
Is becoming a Clinical Research Manager a good career path?
A Clinical Research Manager plays a key role in the healthcare industry. This person oversees clinical trials and research studies. They ensure that everything runs smoothly and follows the rules. It's a job that combines leadership with a deep understanding of medicine and research.
Working in this role offers many benefits. Clinical Research Managers often work with smart, dedicated teams. They get to see their work help develop new treatments. Plus, they can travel to conferences and meet other professionals in the field. However, it also has its challenges. The job can be stressful, with tight deadlines and high stakes. It also requires a lot of education and experience. Managers need strong skills in both research and people management.
Considering the pros and cons can help someone decide if this career is right for them. The rewards include job satisfaction and the chance to make a real impact in healthcare. The challenges involve the pressure and need for ongoing education. It's important to weigh these factors carefully.
Here are some pros and cons to think about:
- Pro: Opportunity to help improve patient care.
- Pro: Potential for career advancement.
- Pro: Collaboration with a skilled team.
- Con: High level of responsibility.
- Con: Demanding work hours.
- Con: Need for continuous learning and certification.
What is the job outlook for a Clinical Research Manager?
Job seekers aiming to become a Clinical Research Manager can find optimism in the job market. According to the Bureau of Labor Statistics (BLS), there are about 6,500 positions available each year. This steady number shows a reliable demand for skilled professionals in this role. With the industry's growth, job seekers can look forward to ample opportunities.
The BLS also projects a 4.8% increase in job openings for Clinical Research Managers from 2022 to 2032. This growth suggests that the field is expanding. This positive outlook indicates a promising future for those entering the profession. With more companies focusing on research and development, the demand for these managers will continue to rise. Job seekers can expect a dynamic and growing career path.
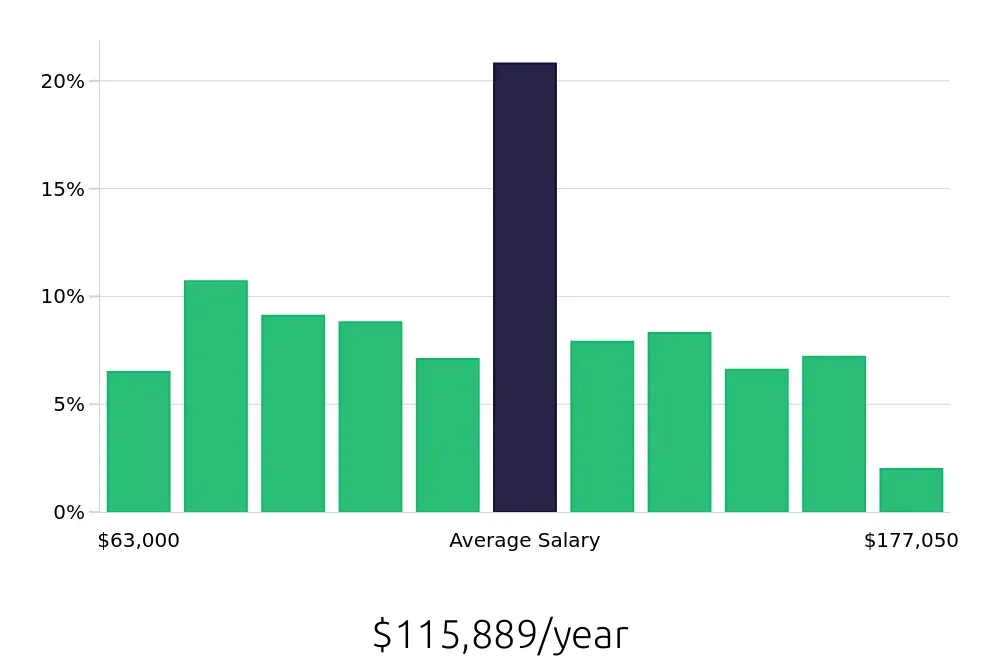
In terms of compensation, Clinical Research Managers earn a robust average of $169,120 annually. This figure reflects the value placed on their expertise and responsibility. On an hourly basis, the average compensation stands at $81.31. Such earnings make this career an attractive option for many. With these figures from the BLS, job seekers can anticipate a rewarding career in terms of both growth and income.
Currently 108 Clinical Research Manager job openings, nationwide.
Continue to Salaries for Clinical Research Manager